PRIMARY ENDPOINT
Time to first bone complication (non-inferiority)
SELECT SECONDARY ENDPOINT
Time to first bone complication (superiority)
Secondary endpoints1,2
If the primary endpoint of non inferiority was met; the superiority test for secondary endpoint was concluded.
Safety and tolerability was also addressed.
OLE, open-label extension; SRE, skeletal-related event.
| Baseline characteristics | XGEVA® (n=2,862) |
ZA (n=2,861) |
| Median age, years | 63 | 63 |
| ECOG status 0-1, % | 90 | 89 |
| Creatinine clearance ≥30 and ≤60 mL/mina, % |
17 | 17 |
| Presence visceral metastases, % | 42 | 40 |
| Prior bone complication, % | 39 | 40 |
| Primary tumor typeb, % | XGEVA® (n=2,862) |
ZA (n=2,861) |
| Breast | 36 | 36 |
| Prostate | 33 | 33 |
| Non-small cell lung | 12 | 12 |
| Multiple myeloma | 3 | 3 |
| Renal | 2 | 2 |
| Small cell lung | 2 | 2 |
| Other | 11 | 10 |
aPatients with creatinine clearance <30 mL/min were excluded from XGEVA® pivotal trials.1
bBased on randomization; total may not equal 100% due to rounding.2
ECOG, Eastern Cooperative Oncology Group.
In a prespecified, integrated analysis

*HR is defined as the increase or decrease in likelihood of an event of interest (in this case, a bone complication) for one group relative
to a comparator group.
CI, confidence interval; HR, hazard ratio.

In women with breast cancer and bone metastases
NCCN Clinical Practice Guidelines (NCCN Guidelines®)
Denosumab (XGEVA® Q4W) is a Category 1† recommended option for the prevention of bone complications in women with breast cancer and bone metastases8
In men with prostate cancer and bone metastases

Denosumab (XGEVA® Q4W) is a Category 1‡ Preferred bone-targeting agent for men with prostate cancer and bone metastases10
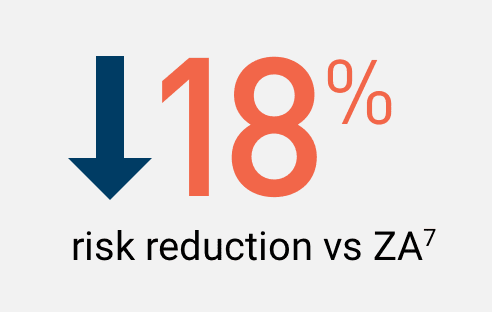
XGEVA® prevented bone complications§ longer vs ZA for patients with other solid tumors metastatic to bone1,11,12
In patients with lung cancer, other solid tumors, or MM,
XGEVA® prevented bone complications§ for a median of 20.5 months which is 4.2 months longer vs ZA (non-inferiority)1,11
Secondary endpoint of superiority was not achieved with the inclusion of MM patients
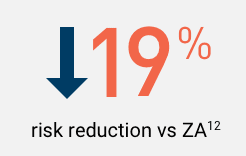
In a post hoc subgroup analysis of patients with lung cancer or other solid tumors, excluding MM,
XGEVA® delayed the time to first bone complications§ for 6 months longer than ZA. Median of 21.4 months for XGEVA® vs 15.4 months for ZA1,12
Time to first bone complication in post hoc subgroup analysis of patients with other solid tumors, excluding MM1,12
§Bone complications, also known as skeletal-related events (SREs), are defined as radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression.1
**Hazard ratio (HR) is defined as the increase or decrease in likelihood of an event of interest (in this case, a bone complication) for one group relative to a comparator group.2,13
Lung cancer patients (39% of the OST/MM population) who received XGEVA® experienced a treatment effect consistent with the OST/MM population12,14
*Results collected from a retrospective study, which included 176 patients with breast cancer and bone metastases from 2008-2012. Bone complications were defined as: pathologic fracture, surgery to bone, radiation to bone, spinal cord compression, and hypercalcemia of malignancy.15
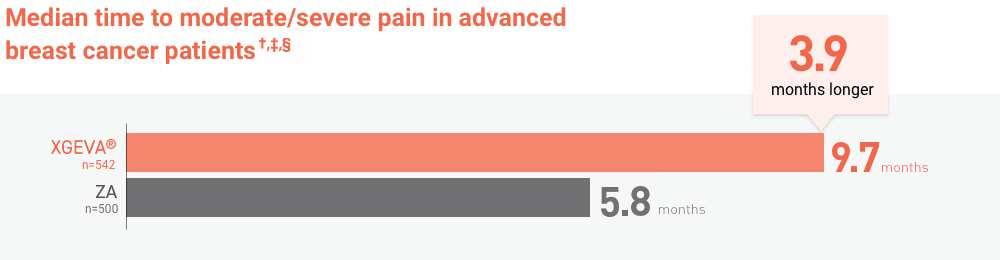

BPI-SF, Brief Pain Inventory (short form).
Pre-existing hypocalcemia must be corrected prior to initiating therapy with XGEVA®. XGEVA® can cause severe symptomatic hypocalcemia, and fatal cases have been reported. Monitor calcium levels, especially in the first weeks of initiating therapy, and administer calcium, magnesium, and vitamin D as necessary. Concomitant use of calcimimetics and other drugs that can lower calcium levels may worsen hypocalcemia risk and serum calcium should be closely monitored. Advise patients to contact a healthcare professional for symptoms of hypocalcemia.
An increased risk of hypocalcemia has been observed in clinical trials of patients with increasing renal dysfunction, most commonly with severe dysfunction (creatinine clearance less than 30 mL/minute and/or on dialysis), and with inadequate/no calcium supplementation. Monitor calcium levels and calcium and vitamin D intake.
XGEVA® is contraindicated in patients with known clinically significant hypersensitivity to XGEVA®, including anaphylaxis that has been reported with use of XGEVA®. Reactions may include hypotension, dyspnea, upper airway edema, lip swelling, rash, pruritus, and urticaria. If an anaphylactic or other clinically significant allergic reaction occurs, initiate appropriate therapy and discontinue XGEVA® therapy permanently.
Patients receiving XGEVA® should not take other denosumab products (e.g., Prolia®).
Osteonecrosis of the jaw (ONJ) has been reported in patients receiving XGEVA®, manifesting as jaw pain, osteomyelitis, osteitis, bone erosion, tooth or periodontal infection, toothache, gingival ulceration, or gingival erosion. Persistent pain or slow healing of the mouth or jaw after dental surgery may also be manifestations of ONJ. In clinical trials in patients with cancer, the incidence of ONJ was higher with longer duration of exposure.
Patients with a history of tooth extraction, poor oral hygiene, or use of a dental appliance are at a greater risk to develop ONJ. Other risk factors for the development of ONJ include immunosuppressive therapy, treatment with angiogenesis inhibitors, systemic corticosteroids, diabetes, and gingival infections.
Perform an oral examination and appropriate preventive dentistry prior to the initiation of XGEVA® and periodically during XGEVA® therapy. Advise patients regarding oral hygiene practices. Avoid invasive dental procedures during treatment with XGEVA®. Consider temporarily interrupting XGEVA® therapy if an invasive dental procedure must be performed.
Patients who are suspected of having or who develop ONJ while on XGEVA® should receive care by a dentist or an oral surgeon. In these patients, extensive dental surgery to treat ONJ may exacerbate the condition.
Atypical femoral fracture has been reported with XGEVA®. These fractures can occur anywhere in the femoral shaft from just below the lesser trochanter to above the supracondylar flare and are transverse or short oblique in orientation without evidence of comminution.
Atypical femoral fractures most commonly occur with minimal or no trauma to the affected area. They may be bilateral and many patients report prodromal pain in the affected area, usually presenting as dull, aching thigh pain, weeks to months before a complete fracture occurs. A number of reports note that patients were also receiving treatment with glucocorticoids (e.g. prednisone) at the time of fracture. During XGEVA® treatment, patients should be advised to report new or unusual thigh, hip, or groin pain. Any patient who presents with thigh or groin pain should be suspected of having an atypical fracture and should be evaluated to rule out an incomplete femur fracture. Patients presenting with an atypical femur fracture should also be assessed for symptoms and signs of fracture in the contralateral limb. Interruption of XGEVA® therapy should be considered, pending a risk/benefit assessment, on an individual basis.
Clinically significant hypercalcemia requiring hospitalization and complicated by acute renal injury has been reported in XGEVA®-treated patients with GCTB and in patients with growing skeletons within one year of treatment discontinuation. Monitor patients for signs and symptoms of hypercalcemia after treatment discontinuation and treat appropriately.
Multiple vertebral fractures (MVF) have been reported following discontinuation of treatment with denosumab. Patients at higher risk for MVF include those with risk factors for or a history of osteoporosis or prior fractures. When XGEVA® treatment is discontinued, evaluate the individual patient’s risk for vertebral fractures.
XGEVA® can cause fetal harm when administered to a pregnant woman. Based on findings in animals, XGEVA® is expected to result in adverse reproductive effects.
Advise females of reproductive potential to use effective contraception during therapy, and for at least 5 months after the last dose of XGEVA®. Apprise the patient of the potential hazard to a fetus if XGEVA® is used during pregnancy or if the patient becomes pregnant while patients are exposed to XGEVA®.
The most common adverse reactions in patients receiving XGEVA® with bone metastasis from solid tumors were fatigue/asthenia, hypophosphatemia, and nausea. The most common serious adverse reaction was dyspnea. The most common adverse reactions resulting in discontinuation were osteonecrosis and hypocalcemia.
For multiple myeloma patients receiving XGEVA®, the most common adverse reactions were diarrhea, nausea, anemia, back pain, thrombocytopenia, peripheral edema, hypocalcemia, upper respiratory tract infection, rash, and headache. The most common serious adverse reaction was pneumonia. The most common adverse reaction resulting in discontinuation of XGEVA® was osteonecrosis of the jaw.
XGEVA® is indicated for the prevention of skeletal-related events in patients with multiple myeloma and in patients with bone metastases from solid tumors.
Please see full Prescribing Information.
Pre-existing hypocalcemia must be corrected prior to initiating therapy with XGEVA®. XGEVA® can cause severe symptomatic hypocalcemia, and fatal cases have been reported. Monitor calcium levels, especially in the first weeks of initiating therapy, and administer calcium, magnesium, and vitamin D as necessary. Concomitant use of calcimimetics and other drugs that can lower calcium levels may worsen hypocalcemia risk and serum calcium should be..